|
|
HSE Walk-In Children’s Flu Vaccine Clinic In Thurles on Tuesday January 20th
Parents and guardians in Thurles and surrounding areas are reminded that a free walk-in flu vaccination clinic for children (ages 2 to 17 years) will take place next week at the Thurles Primary Care Centre on Mitchel Street.
Clinic details: Walk-in – no appointment needed.
Date: Tuesday 20th January
Time: 1:00pm to 6:00pm
Venue: Thurles Primary Care Centre, 22A Mitchel Street, Thurles, Co. Tipperary, (Eircode E41 K4C2).
About the vaccine.
Children aged 2 to 17 can receive the nasal flu vaccine free of charge. The vaccine is given as a spray in the nose, with the vaccinator spraying once into each nostril.
The flu vaccine helps to protect children against flu, and flu can lead to serious complications for some children, including pneumonia.
What to bring.
Please bring ID with your child’s date of birth, such as a passport, birth certificate, Public Services Card or school ID.
Other options.
If you cannot attend the walk-in clinic, your child can also get the flu vaccine through your GP or pharmacist (free for children aged 2 to 17).
Further information.
For general queries, you can contact HSE Live on Freephone 1800 700 700.
Further update to SMA infant formula and follow on formula recall to include batches and products possibly distributed to Ireland from the UK.
Alert Summary dated Tuesday, January 13th 2026.
Category 1: For Action
Alert Notification: 2026.01 (Update 4)
Product Identification: Please see ‘List of batches and products’ in Message section below for product details.
Batch Code: Please see ‘List of batches and products’ in Message section below for batch codes and expiry dates. Please note, batch codes can be found on the base of the tin or box for powdered formulas or the base of the outer box and on the side/top of the container for ready-to-feed formulas.
Message:
Further to FSAI Food Alert 2026.01, FSAI Food Alert 2026.01 (Update 1), FSAI Food Alert 2026.01 (Update 2), FSAI Food Alert 2026.01 (Update 3), the FSAI is notifying consumers of the possible presence of cereulide in additional SMA infant formula and follow on formula batches and products in the list below, due to possible indirect distribution to Ireland from the UK.
See Here: List of affected batches and products.
Recall notices will be displayed at point-of-sale.
Questions and answers.
Nestlé is advising its customers that have purchased any of these batches to contact them via its online form, by sharing a photo of the product and the batch code: www.nestle.co.uk/en-gb/getintouch; or by calling its careline on Tel: 1800 931 832.
Nature Of Danger:
Cereulide toxin is produced by the bacterium Bacillus cereus. The toxin may be pre-formed in a food and is extremely heat resistant. Consumption of foods containing cereulide toxin can lead to nausea and severe vomiting. Symptoms can appear within five hours. The duration of illness is usually 6 to 24 hours.
Action Required: Manufacturers, Wholesalers, Distributors, Caterers & Retailers:
Retailers: Same are requested to remove the implicated batches from sale and display recall notices at point-of-sale.
Wholesalers/Distributors: Same are requested to contact their affected customers and recall the implicated batches and provide a point-of-sale recall notice to their retailer customers.
Consumers: Parents, guardians and caregivers are advised not to feed the implicated batches to infants or young children.
Most significant reform of Irish asylum laws in the history of the Irish State.
The government has given approval to publish the International Protection Bill 2026, legislation that will lead to the “most significant reform of Irish asylum laws in the history of the State” in line with the EU Migration and Asylum Pact.
The Bill will put in place a new EU framework to manage migration and asylum for the long-term and will ensure Ireland’s policy aligns with other EU countries.
The overall objective of the Bill is to provide a fair, sustainable and efficient asylum procedure that is consistent with how asylum laws operate across the EU.
The Bill introduces faster processing of asylum claims with a much more efficient decision-making system. Faster processing will mean that applicants spend less time in IPAS accommodation, and it will significantly reduce the cost of the asylum system to the State. Faster decision-making will also mean that successful applicants will be granted international protection sooner, and those whose applications are refused can be returned to their country of origin sooner.
The International Protection Bill 2026 will replace the International Protection Act 2015.
In July 2025, the Department launched the first phase of pilot pact implementation programme. The first phase aimed to test the ability to process cases end-to-end within the time frame of the future Border Procedure. This requires a first and second-instance decision, with a return order where appropriate, delivered within 12 weeks, and a return effected within a further 12 weeks. During this first phase, the implementation team also mirrored some elements of the screening process as well as parts of the future border procedure that are permitted under current legislation.
Phase one was conducted from July 1st to October 7th 2025 and included applicants from three designated safe countries of origin, Georgia, India and Brazil.
During the initial three months, pilot applicants were successfully processed within the 12 week timeline permitted for first and second instance decisions under the Border Procedure. On average, cases took less than 60 days from application to final decision being issued.
This represents a significant shift from the current median processing times in the IPO and IPAT, and therefore a significant reduction in costs for accommodation and other supports.
On October 8th 2025 the second phase of the transition pilot was launched with the addition of the remaining 12 designated safe countries of origin. Early this year future phases of the pilot will be implemented, in advance of the Pact coming into effect in June 2026.
The government and the Attorney General are developing provisions for inclusion in the Bill to give effect to the proposals, approved by Government on November 26th 2025, that adults who are beneficiaries of international protection will not be entitled to seek family reunification for a period of three years following their grant of international protection.
They must also demonstrate that they are financially self-sufficient. This will be assessed by reference to appropriate income thresholds to be prescribed by the Minister. They will also have to show financial self-sufficiency and not be in receipt of certain social welfare payments or owe money relating to International Protection Accommodation Services (IPAS) payments.
The Government proposes to bring forward amendments at Committee Stage to address Material Reception Conditions, Restrictions of Movement, Detention, Special Reception Needs and Labour Market Access, as required by the EU Reception Conditions Directive.
Other matters to be dealt with by amendments to the Bill during the legislative process include legal counselling, legal advice and legal aid, and matters relating to data sharing.
The Bill will now be presented to the Houses of the Oireachtas and follow the standard parliamentary process over the coming months with a view to enactment in the Spring session, so that it can become operational as required by EU law by June 12th 2026.
The pre-legislative scrutiny report on the General Scheme, including 92 recommendations, was published on December 1st. Some recommendations have been given effect in the published Bill, and others will be considered as the Bill proceeds through the legislative process.
It was with great sadness that we learned of the death, today Tuesday 13th January 2026, of Mrs Margaret Shanahan (née Stokes), Childers Park, Thurles, Co. Tipperary and formerly Graiguemane, Coalbrook, Thurles, Co. Tipperary.
Pre-deceased by her husband John, parents Edmund and Mary, sisters Jo, Mary, and Frances, great-grandson Noah; Mrs Shanahan passed away peacefully following a long illness most bravely borne.
Her passing is most deeply regretted, sadly missed and lovingly remembered by her sorrowing family; loving son Derek, daughter Rhona (Flanagan), grandchildren Nicole, John, Erin, Kayla and Saoirse, great-grandson Finn, daughter-in-law Anna, son-in-law Tom, sisters Esther and Noreen (Tobin), brothers Tom, John and Pat, nephews, nieces, brothers-in-law, sisters-in-law, extended relatives, neighbours and friends.
Requiescat in Pace.
Funeral Arrangements.
The earthly remains of Mrs Shanahan will repose at Hugh Ryan’s Funeral Home, Slievenamon Road, Thurles, (Eircode E41 CP59), on Thursday afternoon, January 15th, from 5:00pm until 7:00pm, before being received into the Church of St Joseph and St Brigid, Bothar-na-Naomh, Thurles at 7:45pm same evening.
Requiem Mass for Mrs Shanahan will be offered on Friday morning, January 16th, at 11:00am, followed by interment, immediately afterwards in St Peter’s Cemetery, Moycarkey, Thurles, Co. Tipperary.
For those persons who would wish to attend Requiem Mass for Mrs Shanahan, but for reasons cannot, same can be viewed streamed live online, HERE.
The extended Shanahan and Stokes families wish to express their appreciation for your understanding at this difficult time, and have made arrangements for those persons wishing to send messages of condolence, to use the link shown HERE.
Ar dheis Dé go raibh a h-anam dílis.
From Ultra-Processed Foods To Hormone Residues: Food Safety, Public Health & Corporate Accountability Collide.
A landmark lawsuit filed by the City of San Francisco against major food and drink manufacturers has signalled a new phase in public health enforcement, one that treats diet-related harm not as an individual failing, but as a market and regulatory failure demanding immediate accountability.
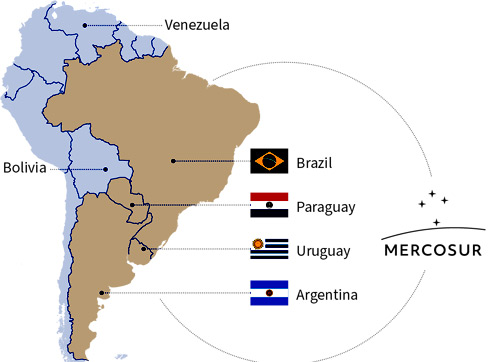
San Francisco alleges that ultra-processed foods were engineered and marketed in ways that encourage over-consumption, especially among children, and that the public ultimately pays the price through higher rates of chronic disease and spiralling healthcare costs. While that case will be tested in court, its wider message is already echoing across the Atlantic: Europe is facing its own “trust test” over what we allow into our food chain, particularly under the EU–Mercosur trade agreement.
Why this matters in Europe now:
On 9 January 2026, EU member states greenlit the signature of the EU–Mercosur agreements, with the European Parliament’s consent still required before conclusion.
The European Commission states that EU rules apply equally to domestic and imported food, and that the agreement “upholds” EU food safety and animal/plant health standards.
However, confidence in “standards on paper” depends on something more basic: verifiable controls and traceability in practice.
Banned substances are not theoretical: recent Irish and EU recalls.
The EU prohibits the use of hormones for growth promotion in farm animals.
EFSA has also noted that ractopamine, a beta-agonist, is banned for use in food-producing animals in the EU and that the ban applies to meat produced in the EU and imported from third countries. Against that backdrop, Irish and EU reporting in recent weeks has documented the recall of Brazilian beef products after banned hormone residues were detected, including confirmation that a quantity entered the Irish market and was subject to official recall and follow-up.
The enforcement gap: what the EU’s own audit found.
A 2024 European Commission DG SANTE audit of Brazil’s residue controls concluded that while many aspects of residue control plans were broadly consistent with EU principles, arrangements to guarantee that cattle destined for the EU market had never been treated with oestradiol 17β were “ineffective”. The audit stated the competent authority could not guarantee the reliability of operators’ sworn statements on non-use, and was not in a position to reliably attest to compliance with the relevant EU health certificate section.
This is the crux of the Mercosur anxiety: not whether Europe has rules, but whether Europe can consistently verify compliance, when supply chains are long, oversight differs, and commercial incentives are strong.
Ultra-processed foods and “addictive design”: the parallel problem.
The San Francisco case centres on claims of deceptive marketing and products engineered to drive consumption.
Meanwhile, the health evidence base around UPFs continues to expand. A major BMJ umbrella review reported that greater UPF exposure is associated with higher risk of adverse health outcomes, particularly cardiometabolic outcomes, across many studies.
Controlled research has also shown that ultra-processed diets can increase calorie intake and weight gain compared with minimally processed diets under tightly controlled conditions.
The common thread is accountability: when products (or supply chains) are designed to maximise throughput and profit, public health cannot rely on consumer vigilance alone.
Calls to action
Tipperary is now calling for a joined-up response that protects consumers, supports credible producers, and restores trust in our food chain:
(1) A tougher “trust-but-verify” regime on imports).
Full use of the EU’s Official Controls framework to ensure import compliance is proven through audits, sampling, and enforceable consequences, not assurances alone.
(2) Mandatory transparency on audit findings and corrective action plans.
Where EU audits identify weaknesses in residue controls or traceability, the public must see timelines, milestones and proof of remediation.
(3) Stronger protections for children in the food environment.
Restrictions on marketing tactics that normalise high-sugar, high-salt, heavily engineered foods to children—mirroring the direction of the San Francisco action.
(4) Clearer front-of-pack information and health claims enforcement.
Consumers should not need a chemistry degree to understand what they are buying, or whether “healthy” claims stand up.
(5) A level playing field for farmers and processors meeting EU rules.
Irish and EU producers operating under strict bans and controls must not be undercut by imports where verification is demonstrably weaker.
San Francisco has drawn a line under the era of ‘hands off’ regulation when public health harms are foreseeable and widespread. Europe is now at a similar crossroads. The EU–Mercosur debate cannot be reduced to tariffs and quotas: it is also about trust, enforcement and the credibility of our bans on hormones and other restricted substances. Public health must not be negotiated away, nor should consumers be asked to carry the risk.
|
Support Us Help keep Thurles.info online by donating below. Thank you.
Total Donated 2026: €40.00
Thank You!
Daily Thurles Mass Livestream
|





Recent Comments